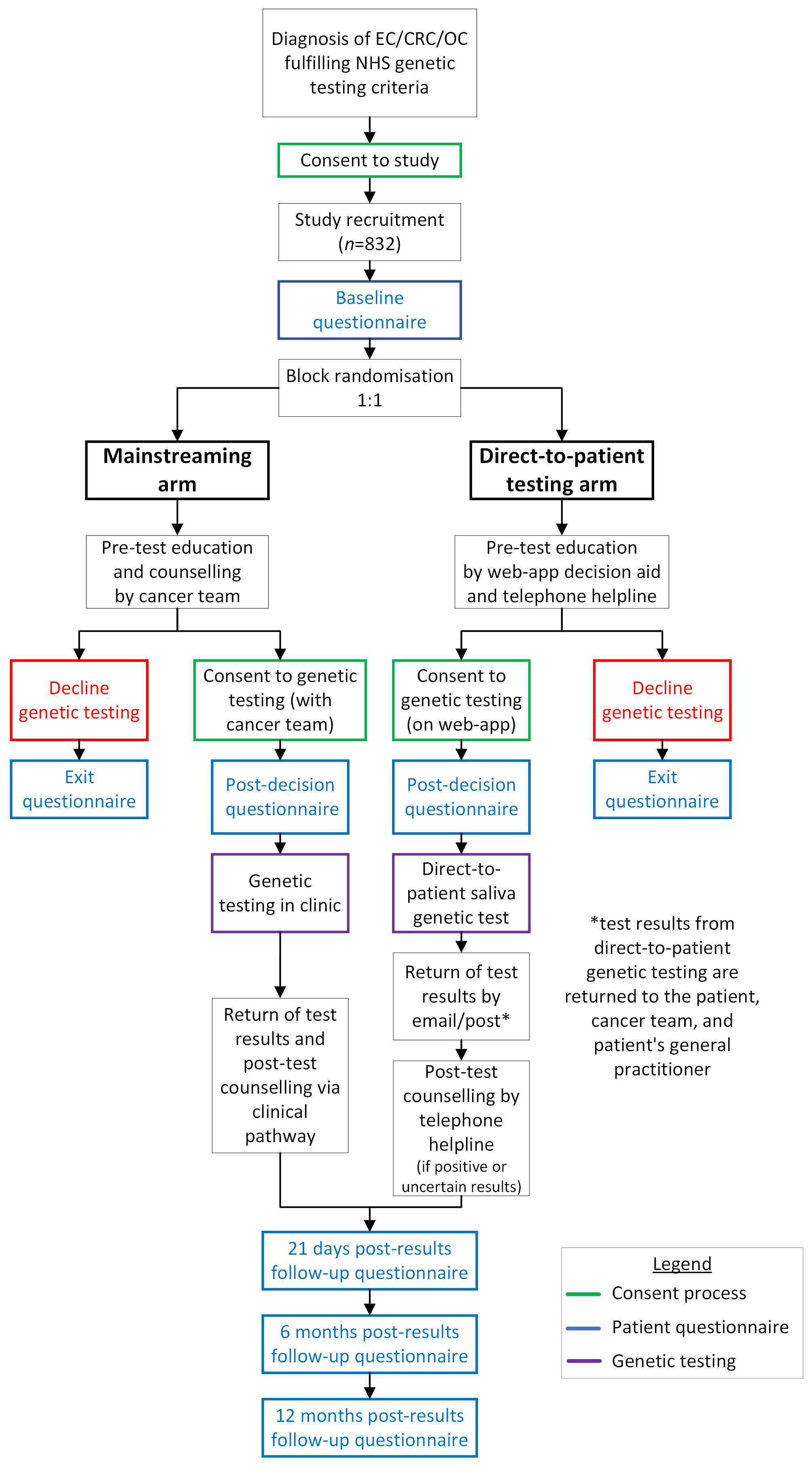
Study design
DETECT-2 is an equivalence two-arm randomised control trial. Eligible patients will be randomised at study recruitment to one of the two arms:
Direct-to-patient testing: Patients will be provided information about genetic testing on a web-app decision aid. This decision aid will be accessible on smartphones, tablets, and web browsers and provide information about genetic testing, how it is performed, the possible results, and potential impacts. The web-app will include the consent form for genetic testing allowing patients to accept or decline genetic testing electronically.
Patients consenting to genetic testing will receive a sample collection kit in the post, provide their sample at home using the saliva-collection kit, and post it back to the genetic testing laboratory to perform the testing.
Results will be returned by post and email, and patients will be supported throughout the entirety of this pathway by a telephone helpline staffed by study counsellors experienced in genetic counselling. Patients with positive or uncertain results from genetic testing will be given post-test counselling. Participating sites will refer these patients to their local genetics service(s) as per standard NHS practice/care followed.
Standard of care: Patients will receive pre-test counselling from a non-genetics clinician with consent for genetic testing, blood sample collection, and return of results undertaken in clinic as per routine hospital practice.
Hypothesis: A direct-to-patient approach of unselected genetic testing for colorectal, endometrial, and ovarian cancer is equivalent in terms of uptake of genetic testing when compared to a traditional non-genetic clinician based mainstreaming approach.
Primary outcome: Uptake of genetic testing.
Secondary outcomes:
Decision satisfaction or regret
Mental health and emotional outcomes
Participant quality-of-life
Attitudes, experiences, and impact on emotional health
Decision aid and telephone helpline use in direct-to-patient testing pathway
Variant prevalence
Cost-effectiveness of both testing approaches
Clinician experience of direct-to-patient testing pathway
Outcomes will be assessed by the following methods:
Evaluating uptake of genetic testing in both arms
Patient questionnaires administered at the following time points:
Baseline (at consent to the trial)
Post-decision to accept or decline genetic testing
21-days after return of genetic test result
6 months after return of genetic test result
12 months after return of genetic test result
Qualitative interviews with participants following genetic testing
Evaluation of web-app decision aid and telephone helpline usage
Collection of costing data in both arms from a health service perspective and undertaking cost-effectiveness analysis comparing Direct-to-Patient testing with mainstreaming genetic testing.
Clinician opinion questionnaires
Sample size: 832 patients need to be recruited to assess the primary hypothesis